Revolutionary PERT. The human genome is often compared to a massive, intricate instruction manual. It contains the precise recipes for building every protein required to keep us alive and functioning.
But what happens when a typo occurs in that manual?
Specifically, what happens when a period is placed accidentally in the middle of a crucial sentence, causing the reader to stop reading before the instruction is complete?
Revolutionary PERT.
In genetics, this specific type of “typo” is known as a nonsense mutation. For decades, these mutations have plagued scientists and doctors alike, causing severe, often fatal, hereditary diseases.
However, a groundbreaking new tool known as PERT (Prime Editor-mediated Read-through of Premature termination codons) has emerged from the laboratories of the Broad Institute, offering a glimmer of hope where previously there was little.
This isn’t just another incremental step in medicine; it is a potential paradigm shift. PERT represents a versatile, “Swiss Army knife” approach to gene editing that could theoretically cure a vast array of genetic disorders by teaching the body to ignore the errors written in its own code.
Revolutionary PERT, “Stop Sign” Problem.
Understanding Nonsense Mutations.
To appreciate the brilliance of PERT, one must first understand the enemy it is fighting. In a healthy cell, the machinery responsible for building proteins reads the DNA instructions until it hits a specific signal a “stop codon” which tells it that the protein is finished.
A nonsense mutation creates a fake stop codon in the middle of the gene sequence. When the cellular machinery encounters this premature red light, it halts production immediately. The result is a truncated, incomplete protein that is usually broken down by the cell as trash.
Why this matters?
Nonsense mutations are responsible for nearly 25% of all known genetic diseases. From Cystic Fibrosis to Duchenne Muscular Dystrophy, these premature stop signals rob the body of essential tools it needs to survive.
Until now, fixing these errors has been like trying to repair a single broken brick in a massive wall without disturbing the structure. But PERT changes the game by introducing a way to bridge the gap.
How PERT Works.
The “Bridge” Over the Broken Code.
The technology, detailed in a landmark study published in the journal Nature, was spearheaded by David Liu, a renowned chemical biologist at the Broad Institute in Cambridge, Massachusetts. Liu and his team are already famous for pioneering “prime editing,” a precise method of rewriting DNA. With PERT, they have taken this a step further.
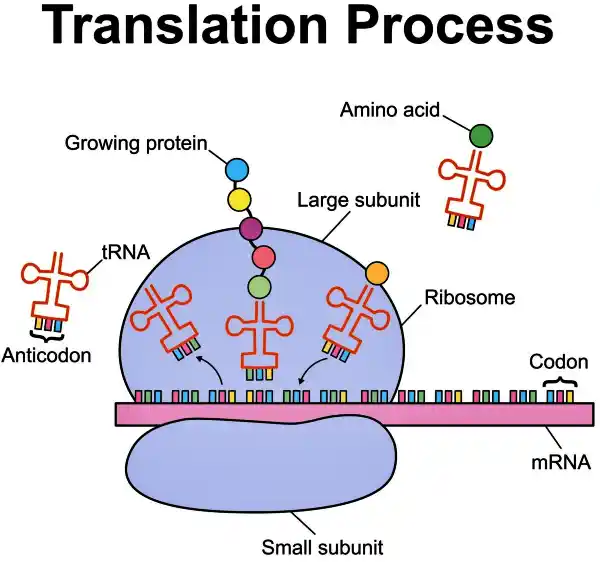
The PERT method is a sophisticated hybrid. It combines prime editing with synthetic suppressor tRNAs.
1. The Architect (Prime Editing): The system uses prime editing to insert a new instruction into the cell’s genome.
2. The Messenger (Suppressor tRNA): This new instruction tells the cell to manufacture a specific type of transfer RNA (tRNA).
3. The Fix: Normally, tRNAs help build proteins by bringing amino acids to the assembly line. The suppressor tRNA introduced by PERT is special it is designed to recognize the “fake” stop sign caused by the mutation. Instead of stopping, it slots in an amino acid and allows the protein synthesis to continue right past the error.
In essence, PERT programs the cell to say, “I see a stop sign here, but I know it’s a mistake, so I’m going to keep driving.” By doing so, the cell produces a full-length, functional protein.
Proof of Concept.
Success in Human Cells.
The theoretical elegance of PERT is matched by its practical success in laboratory trials. Liu’s team tested the technology on cultured human cells carrying devastating genetic mutations. The results were striking. PERT successfully corrected the cellular defects associated with several severe conditions:
• Cystic Fibrosis: A disease that damages the lungs and digestive system.
• Batten Disease: A fatal disorder of the nervous system that begins in childhood.
• Tay-Sachs Disease: A rare disorder that destroys nerve cells in the brain and spinal cord.
• Niemann-Pick Disease: A metabolic disorder causing harmful accumulation of lipids in organs.
In each case, the introduction of PERT allowed the cells to “read through” the mutation and produce the vital proteins that were previously missing.
Moving to Mammals.
The Mouse Study.
While cellular studies are promising, the true test of any gene therapy is how it functions in a living organism. To validate PERT, the researchers utilized a mouse model engineered with a nonsense mutation that mimics Hurler syndrome, a severe human condition caused by a lack of the IDUA enzyme.
The results, while realistic, were profoundly encouraging.
The treatment did not restore protein levels to 100%. Instead, PERT restored the production of the full-length IDUA protein to roughly 7.6% of normal levels. While this number might seem low to a layperson, in the world of genetics, it is often a victory.
The Threshold Effect.
For many genetic diseases, the body doesn’t need perfect protein levels to function. It just needs enough. In the case of the mice, that 7.6% was sufficient to significantly alleviate the symptoms of the disease.
This suggests that PERT doesn’t need to be perfect to be a life-saver; it just needs to be effective enough to push the patient past the threshold of survival.
Why PERT Beats the Competition.
Zoya Ignatova, a biochemist at the University of Hamburg who was not involved in the study, has praised the approach as highly promising. She highlights that while other researchers have experimented with suppressor tRNAs, PERT solves the delivery problem in a unique way.
The “One-and-Done” Advantage.
Previous attempts to use suppressor tRNAs relied on delivering them via lipid nanoparticles (similar to the COVID-19 mRNA vaccines) or viral vectors.
• Lipid Nanoparticles: These are temporary. The patient would need repeated injections for the rest of their life to maintain protein levels.
• Viral Vectors: These can trigger dangerous immune reactions, limiting how much therapy a patient can receive.
PERT is different. Because it uses prime editing to actually insert the gene coding for the suppressor tRNA into the patient’s own DNA, the cell adopts the cure permanently. Theoretically, a single treatment could provide a lifetime supply of the corrective molecule.
Disease-Agnostic Potential.
Most gene therapies are bespoke—custom-designed for one specific patient’s mutation. PERT has the potential to be a “platform technology.” A single suppressor tRNA could theoretically fix the same type of stop mutation across hundreds of different diseases.
The Challenges Ahead.
Not a Magic Wand Yet.
Despite the excitement, the road to the clinic is long. There are significant biological hurdles that must be cleared before PERT can be used in humans.
• The “Goldilocks” Dilemma: The amount of tRNA matters. If the expression is too low, the disease isn’t treated. If it’s too high, the cell might start ignoring real stop signs at the end of healthy genes, creating abnormally long proteins that could be toxic.
• Tissue Specificity: The study noted that the optimal amount of tRNA varies from tissue to tissue. A dose that works in the liver might be ineffective in the brain, or toxic in the kidneys. Therapies will need to be carefully adapted for different organs.
• Specificity: A specific suppressor tRNA will not work for all nonsense mutations. Different mutations create different “fake” stop signals, requiring a library of different PERT agents.
The Future.
A Frozen Library of Cures.
The vision for PERT is nothing short of cinematic. If the method proves safe and effective in humans, it could dramatically slash the cost and time required to develop treatments for rare diseases.
Currently, developing a gene therapy for a rare condition takes years and millions of dollars. With PERT, researchers envision a future where medical centers might stock a “frozen library” of PERT agents.
When a baby is born with a genetic disease caused by a nonsense mutation, doctors wouldn’t need to invent a new drug from scratch. They would simply sequence the baby’s DNA, identify the type of mutation, and pull the corresponding PERT agent off the shelf.
David Liu and his colleagues are already planning the next phase: developing a wider range of suppressor tRNAs to cover more mutation types and contexts.
While “off-the-shelf” genetic cures may still be years away, the PERT technology proves that the biological possibility exists.
We are moving from an era where we could only read the book of life, to an era where we can edit out the typos that cause suffering, ensuring that for many, the story doesn’t have to end prematurely.
Have a Great Day!